PURTEQ believes cleaning should never be harmful. Our formulas were developed to provide a safer alternative to commonly used cleaning products that contain harsh chemicals like bleach, alcohol and quats. In this paper we will learn the science behind KLENZ Multi-Purpose Cleaner. The active cleaning agent in KLENZ is a supramolecular assembly (group of molecules held together by non-covalent bonds) known as a micelle.
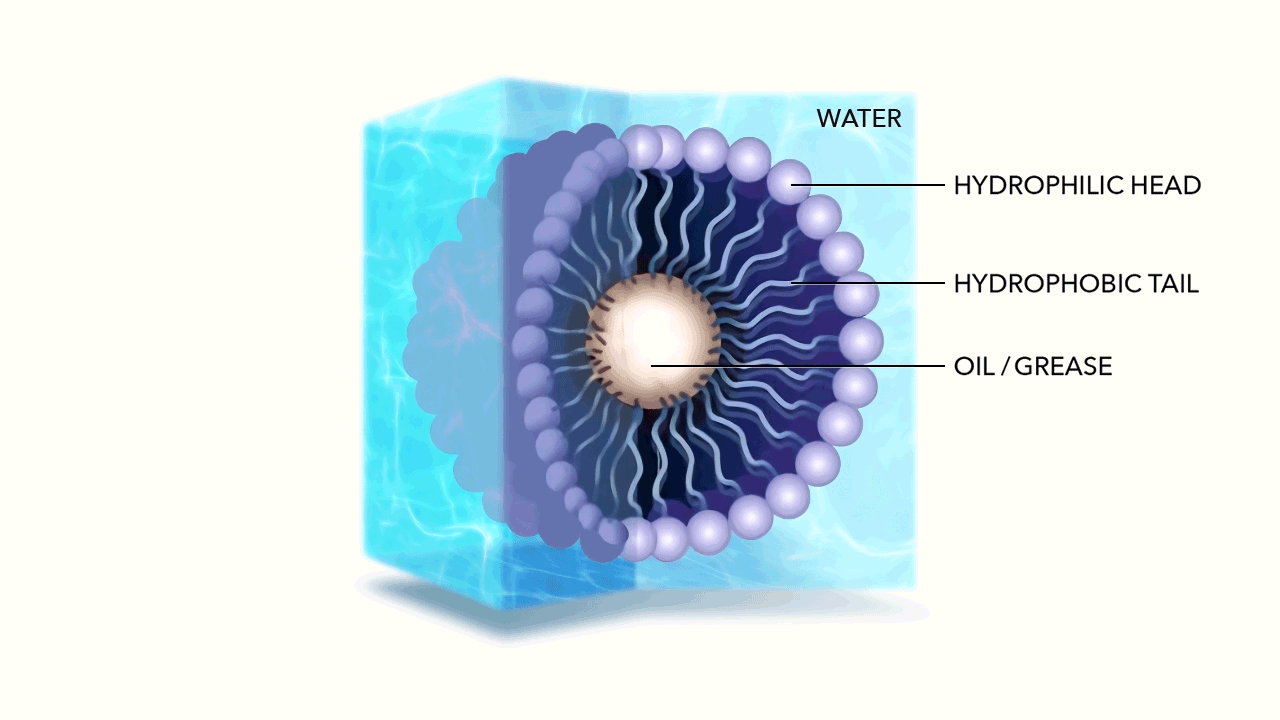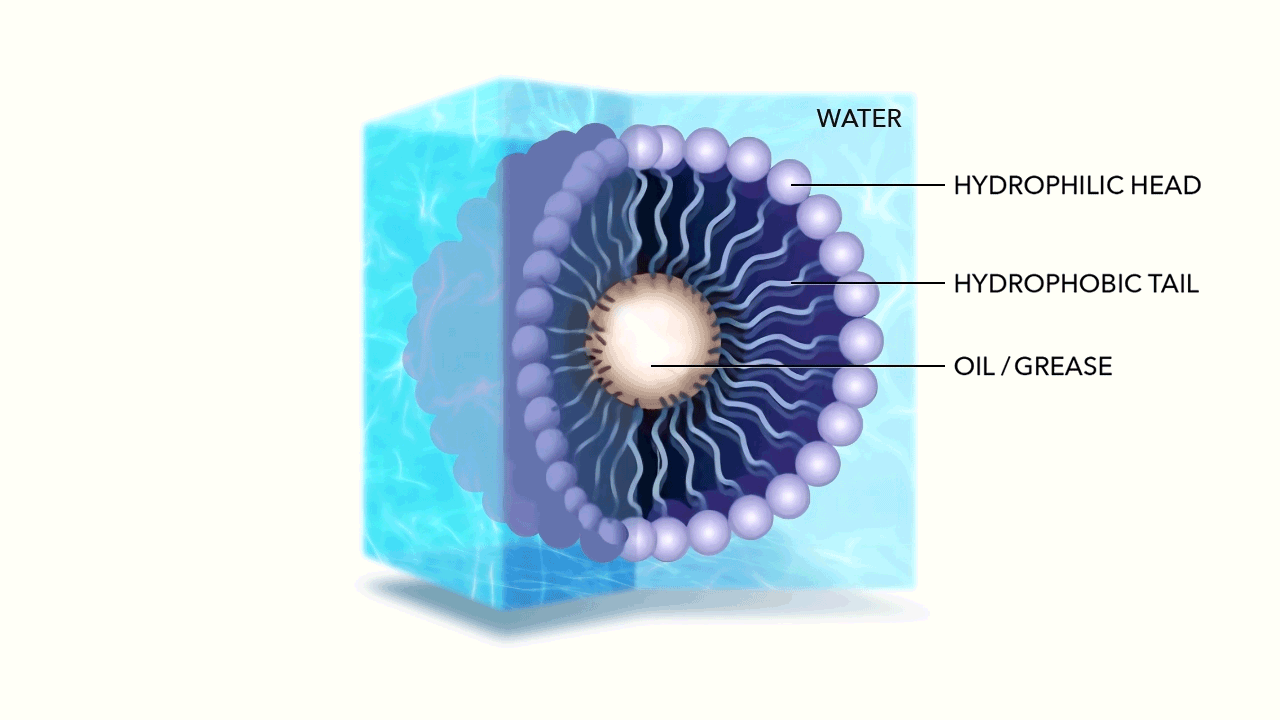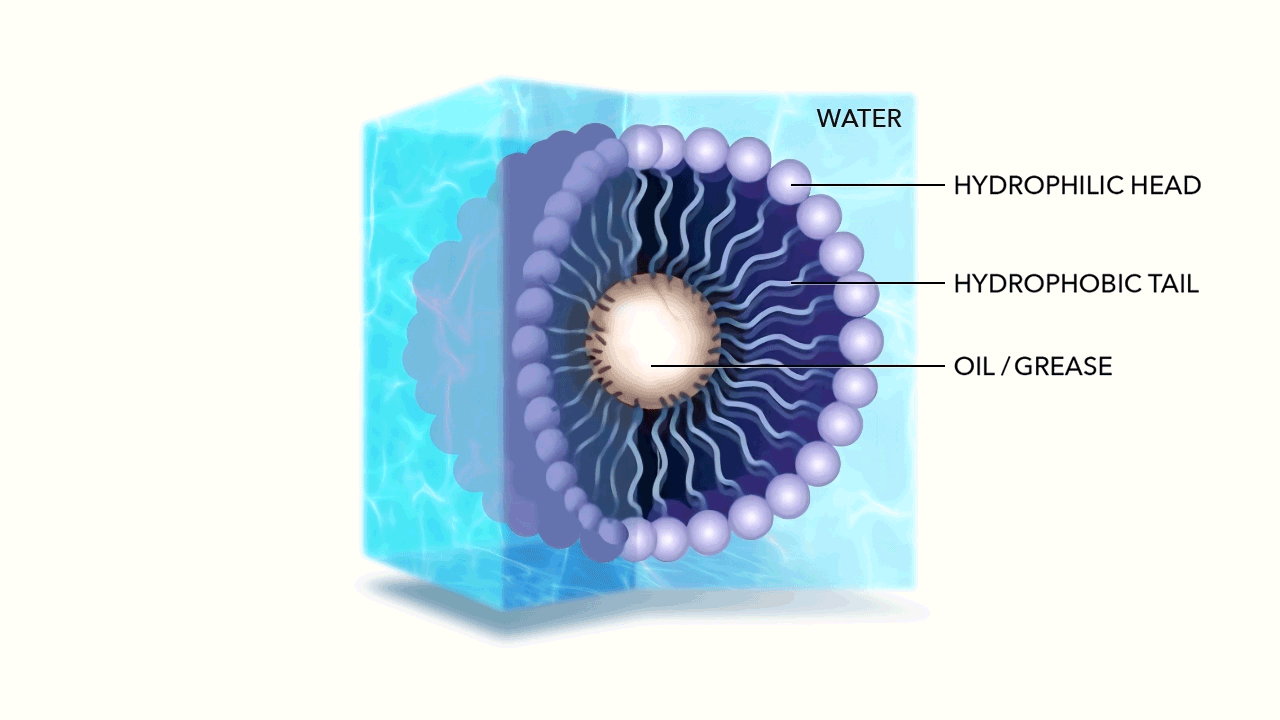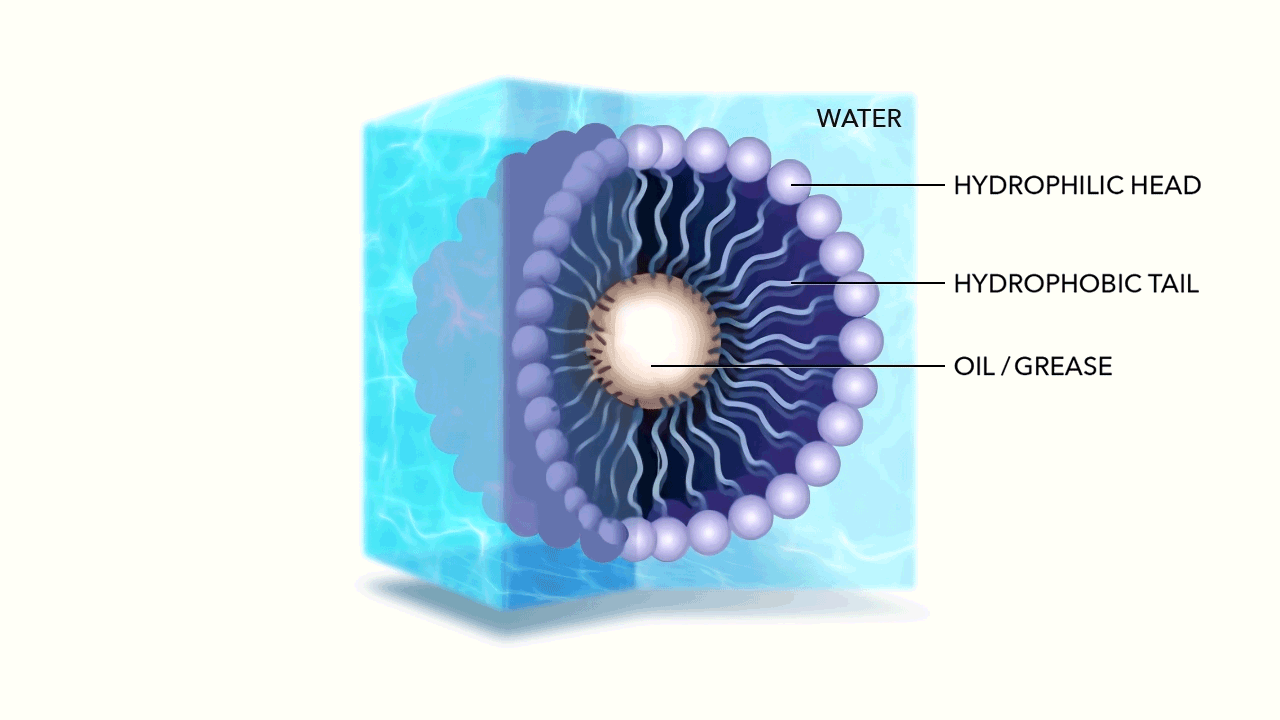
The micelle nanoparticle is composed of lipids. Each individual lipid has two components, a head and a tail. Each end of the lipid reacts differently to water.

Water is a polar molecule, which is to say that the negative and positive charges are not evenly distributed. It is sometimes referred to as a "bent" molecule. Each water (H2O) molecule is made up of two Hydrogen atoms and one Oxygen atom. The Oxygen atom has two orphan electrons. These electrons repel one another. In so doing, they push the Hydrogen atoms to one side (hence "bent"). As a result—though water itself has neither a positive nor negative charge—the positive and negative charges within it are kept at opposite sides of the molecule. This polarity effects its interaction with other substances, including lipids (and their supramolecular assembly, as a micelle).

Unlike the water molecule, the lipid head is negatively charged. Made of phosphates, it is attracted to the positive side of the otherwise neutral, yet polar water molecule. This makes the head of the Lipid hydrophilic (literally "water loving"). The head of the Lipid therefore chases water molecules, attempting to bond with their positive side.
The tail of the Lipid is made of fatty acid and has no charge. This makes the tail hydrophobic (literally "water fearing”) as well as oleophilic or oil loving.
 As the hydrophilic head of the Lipid chases the water molecules, they accumulate, while the tails of the Lipids are repelled, pulling away from the water. Due to their geometry—the head of each Lipid is much larger than its tail—the cluster begins to curve until it forms a sphere, with the water loving head on the exterior, and the water fearing tail on the interior. Spherical micelles are favored entropically, as they are the most stable self assembly. This is referred to as the micelle's "packing characteristic."
As the hydrophilic head of the Lipid chases the water molecules, they accumulate, while the tails of the Lipids are repelled, pulling away from the water. Due to their geometry—the head of each Lipid is much larger than its tail—the cluster begins to curve until it forms a sphere, with the water loving head on the exterior, and the water fearing tail on the interior. Spherical micelles are favored entropically, as they are the most stable self assembly. This is referred to as the micelle's "packing characteristic."
When mixed with sufficient amount of water, this reaction is spontaneous, and known as micellisation.
Chemically, these machinations are taking place at the molecular scale. A micelle is one to four nanometers in diameter. For a point of reference, a human hair is about 80,000 nanometers.

Given the negative charge of the exterior micelle head, the individual micelles repel from one another. Energetically bouncing around, ricocheting off of one another, yet hyper-mobile within water, the membrane created by the Lipid allows hydrophobic molecules (Fats, Oils, and Greases) to easily pass through, being captured within by the oleophilic tails.

The micelles hold these particles in suspension, breaking down their long chain hydrocarbon bonds when they react with the fatty acids in the tail.
This process allows particles that don’t like water, to dissolve within water. The soil constituents resulting from this reaction—nitrate, dissolved oxygen, carbon dioxide, biomass—are fully biodegradable, and easily rinse away, taking the stain along with it.
Learn more about KLENZ at our case studies video, or our product brochure.
Sources:
• The Basics of General, Organic, and Biological Chemistry
Chapter 17.3 Membranes and Membrane Lipids
• The Fact Factor: Associated Colloids (Micelles) by Hemant More
• Molecular Interactions and the Behaviors of Biological Macromolecules by Loren Dean Williams
• Why is the head hydrophilic by Guillaume Boivin
• Formation of Self-Assembling Structures: Micelles, Bilayers, and Vesicles by Kevin Fisher
• ThoughtCo: Why Is Water a Polar Molecule? by Anne Marie Helmenstine, Ph.D.
• Challenges and breakthroughs in recent research on self-assembly, by Katsuhiko Ariga, et al
• Consilience Journal: Micellisation, by Michael Leach
• Colloidal Chemistry Technology, by Kaylin D’Aire
• Compendium of Chemical Terminology, by McNaught & Wilkinson


